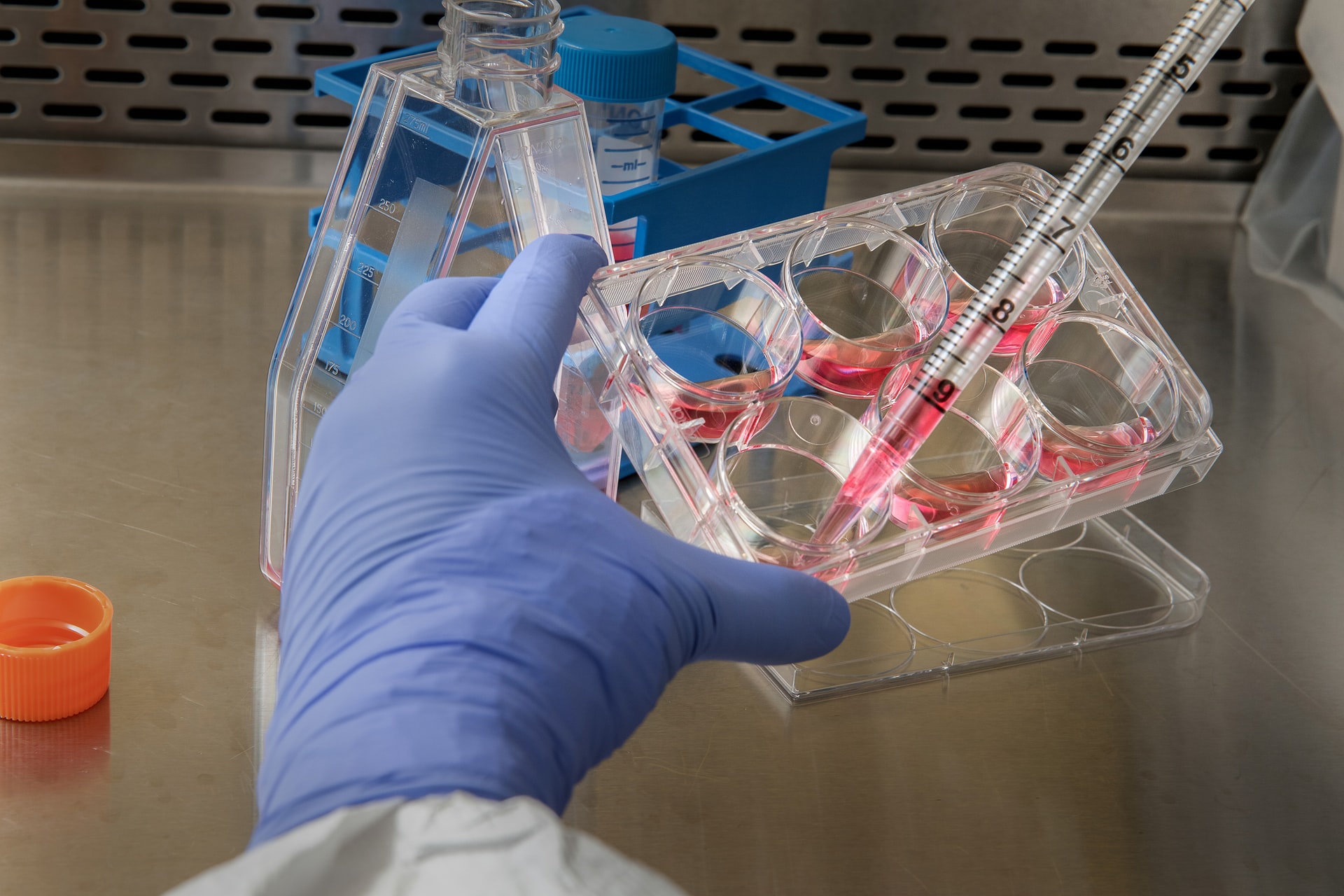
Treatments utilizing Clustered Regularly Interspace Short Palindromic Repeats (CRISPR), continue to rack up clinical success. The latest research on blood disorders like sickle cell disease and beta thalassemia shown that CRISPR products could provide a functional cure just months after initiation of treatment. In other labs, the use of CRISPR has proven effective in combatting nearly all strands of Coronavirus, including SARS-COV2, the cause of COVID-19.
Related ETF: ARK Genomic Revolution Multi-Sector ETF (ARKG)
Curing Blood Disorders
CRISPR Therapeutics AG and Vertex made headlines last Friday when they announced a trio of patients, two with beta thalassemia and one with severe sickle cell disease, saw benefits from one-time treatment with their experimental CTX001. After a follow-up of five to 15 months, all three patients are free from their once-routine need for blood transfusions and painful events that came with their disorders.
“The preliminary results… demonstrate, in essence, a functional cure for patients with beta thalassemia and sickle cell disease,” team member Haydar Frangoul at Sarah Cannon Research Institute in Nashville, Tennessee, said in a statement.
Per Bloomberg, the treatment works by editing genes found inside blood stem cells that suppress the production of hemoglobin made by newborn babies. In this trial, bone marrow stem cells are removed from people and the gene that turns off fetal hemoglobin production is disabled with CRISPR. The remaining bone marrow cells are killed by chemotherapy, then replaced by edited cells. This is done to ensure that new blood cells are produced by the edited stem cells, but the chemotherapy can have serious side effects including infertility.
The biotech companies have opened up enrollment to a broader group of patients. In total, five patients with beta thalassemia and two with sickle cell disease have received treatment so far. The study runs for two years in total, Vertex spokeswoman Heather Nichols told IBD. “Each trial can enroll up for 45 patients,” she said. “The trial is ongoing and there’s been strong demand from both patients and the medical community, so we’re pleased with the progress so far.”
Combating Genetic Blindness and Glaucoma
MRP has been following Vertex and Crispr’s partnership on CTX001 since 2018. Over that same period, we’ve been tracking the progress of Editas Medicine’s groundbreaking CRISPR treatment that could potentially reverse some forms of gene-induced blindness…
To read the rest of this Market Insight, START A FREE TRIAL You’ll also gain access to: If you already have a subscription, sign in